Why is Desalination Important?
About 71% of the Earth is covered in water. While most of that water is found in the ocean, about 2.5% of it is freshwater, which can be used to drink or irrigate crops. However, as climate change begins introducing erratic weather patterns, resulting in lower rain and snowfall, thinner rivers, and drier lakes, the availability of freshwater required to sustain a growing population is becoming a matter of urgent concern.
Today, there are increasing worries that many communities are at risk of losing access to fresh water. There are already 1.1 billion people dealing with water scarcity, and up to 2.7 billion people don’t have access to enough water for at least one month out of the year. Water scarcity has profound humanitarian and economic consequences. It’s not as simple as people simply dying of thirst; a lack of adequate fresh water can lead to declining food production. Another consequence of water scarcity is inadequate sanitation which can cause the spread of water-borne illnesses like cholera and typhoid fever.
The supply of available freshwater, already limited, may continue to decline in the future if droughts become more common. On the other hand, demand for freshwater is likely to continue to rise as the global population is projected to grow from 8 billion in 2023 to nearly 10 billion by 2050. Preventing widespread water insecurity will require a new source of freshwater supply.
What is Desalination?
Desalination is the process of converting the plentiful supply of saltwater in the world’s oceans and seas into usable freshwater. Some countries, notably those in the Middle East, are already relying on desalination processes to supply the lion’s share of their drinking water. Others, like the US, have yet to scale desalinated water production. However, with renewable energy sources like solar and nuclear making energy cheaper and more abundant, alongside desalinating technology becoming more efficient, seawater desalination is likely to become an ever more important source of freshwater around the globe.
Freshwater Distribution and Civilization
All early civilizations were built on the banks of rivers. Whether it was the Euphrates in the Fertile Crescent or the Tiber in Rome, rivers gave early settlements easy access to abundant streams of fresh water, essential not only for drinking but also for irrigating crops. The availability of water was one of the biggest constraints on the growth of settlement and population size. The layout of Ancient Egypt reflected this. It stretched out like a long snake, hugging the banks of the Nile River.
The invention of aqueducts by the Romans first enabled water to be carried long distances, providing the crucial utility to remote stretches of its sprawling empire and allowing populations to settle far from fixed sources of water. The Roman method of diverting freshwater to new habitations is still the essential means modern cities use to provide water to their residents. It’s how the United States built sprawling urban oases in dry deserts like Arizona and Southern California.
Source: Pacific Institute
Freshwater in the United States
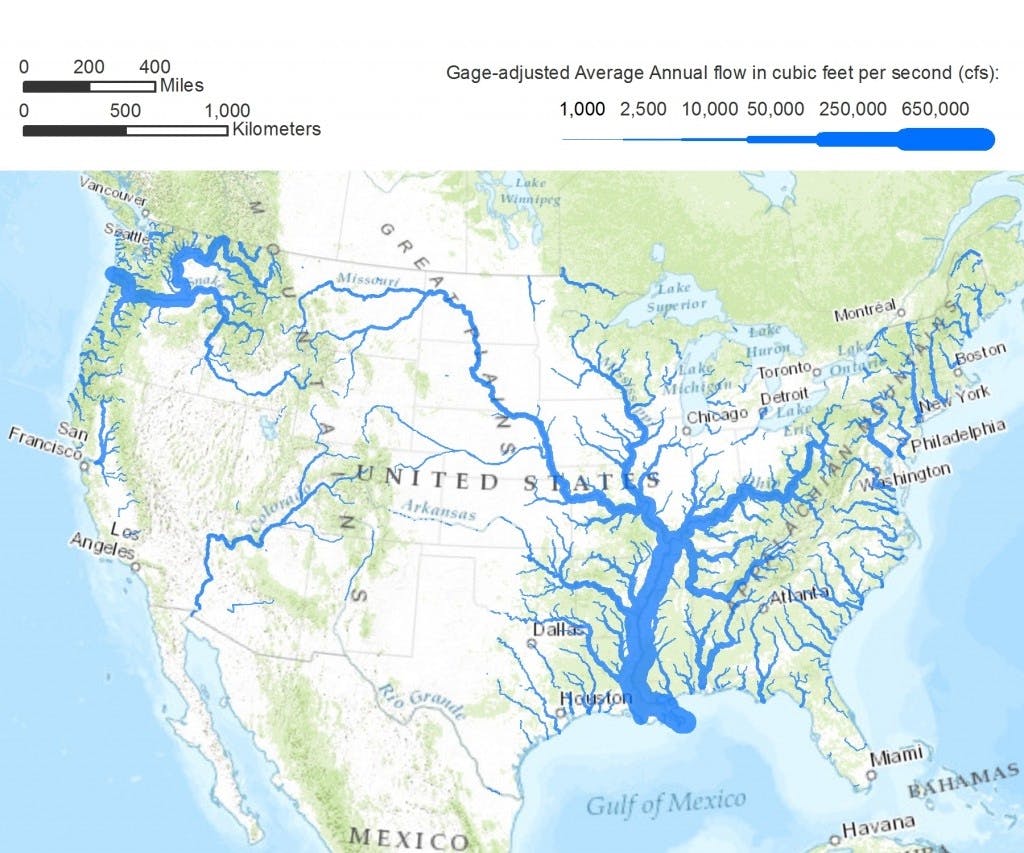
The United States relies on a sprawling network of over 2 million miles of underground pipes and over 153,000 different public water systems to supply the country with over 300 billion gallons of water each day. Though about a quarter of the water used in the United States comes from groundwater, typically found in aquifers that run below the surface, the vast majority (roughly 70%) of the water in this system is sourced from surface water, which includes natural rivers, streams, and reservoirs.
While the United States is geologically blessed to have one of the highest concentrations of lakes and rivers in the world, this bounty is not equitably distributed. While the Eastern United States benefits from access to the plentiful Great Lakes and the colossal Mississippi River, the Southwest is particularly barren.
In that region, the main source of surface water is the Colorado River, which is replenished by snowmelt from the Rocky Mountains. It runs through seven states, including Colorado, Utah, Wyoming, New Mexico, Arizona, California and Nevada. Each of them draws from it as a source of freshwater.
The states closest to the source of the river have an advantage over those further away. If states like Colorado, Utah, and Wyoming drew as much water as they wanted, there would be none left for residents to draw in California and Arizona. The threat of this prompted the signing of the Colorado River Compact in 1922, an agreement that placed a limit on how much water could be extracted by states in the Upper Basin of the River such that enough would be left over for states in the Lower Basin.
For states like California, the agreement was a boon. It opened up a massive new source of guaranteed water supply, which in due time effectively terraformed California into one of the most productive pieces of agricultural land in the US. It also allowed California’s urban hubs like Los Angeles to continue their expansion, which previously had to rely on extreme measures to supply water to their residents.
For example, in a particularly egregious period of water scarcity in the first decade of the 1900s, Los Angeles quietly began buying up water rights around the neighboring Owens Valley, a rural farming community that depended on Sierra Mountain runoff for irrigation. The city then built the Los Angeles aqueduct to ferry the water from Owens Valley, effectively destroying the community’s farming prospects. Over the ensuing years, California was mired in what were called the “California Water Wars” as aggravated Owens Valley residents dynamited the Los Angeles aqueduct, though it was ultimately rebuilt and the Owens Valley community disintegrated after the collapse of their economy.
Today, the Los Angeles Aqueduct still supplies a third of all the water in Los Angeles, but the Colorado River Aqueduct supplies the lion’s share. Over half of the 191 billion gallons Los Angeles consumes annually comes from the Colorado River. However, this once again puts Los Angeles’ future water supply in a precarious position. Over the past 20 years, the Colorado River has lost over 10 trillion gallons of water due to less frequent rain and snowfall caused by climate change. The river’s flow is expected to decrease by over 20% over the next 40 years, even while the population of Southern California keeps growing.
Luckily for the Southwest, this problem already has a technological solution, one that already works at scale in other parts of the world — it’s called desalination.
Freshwater in the Middle East
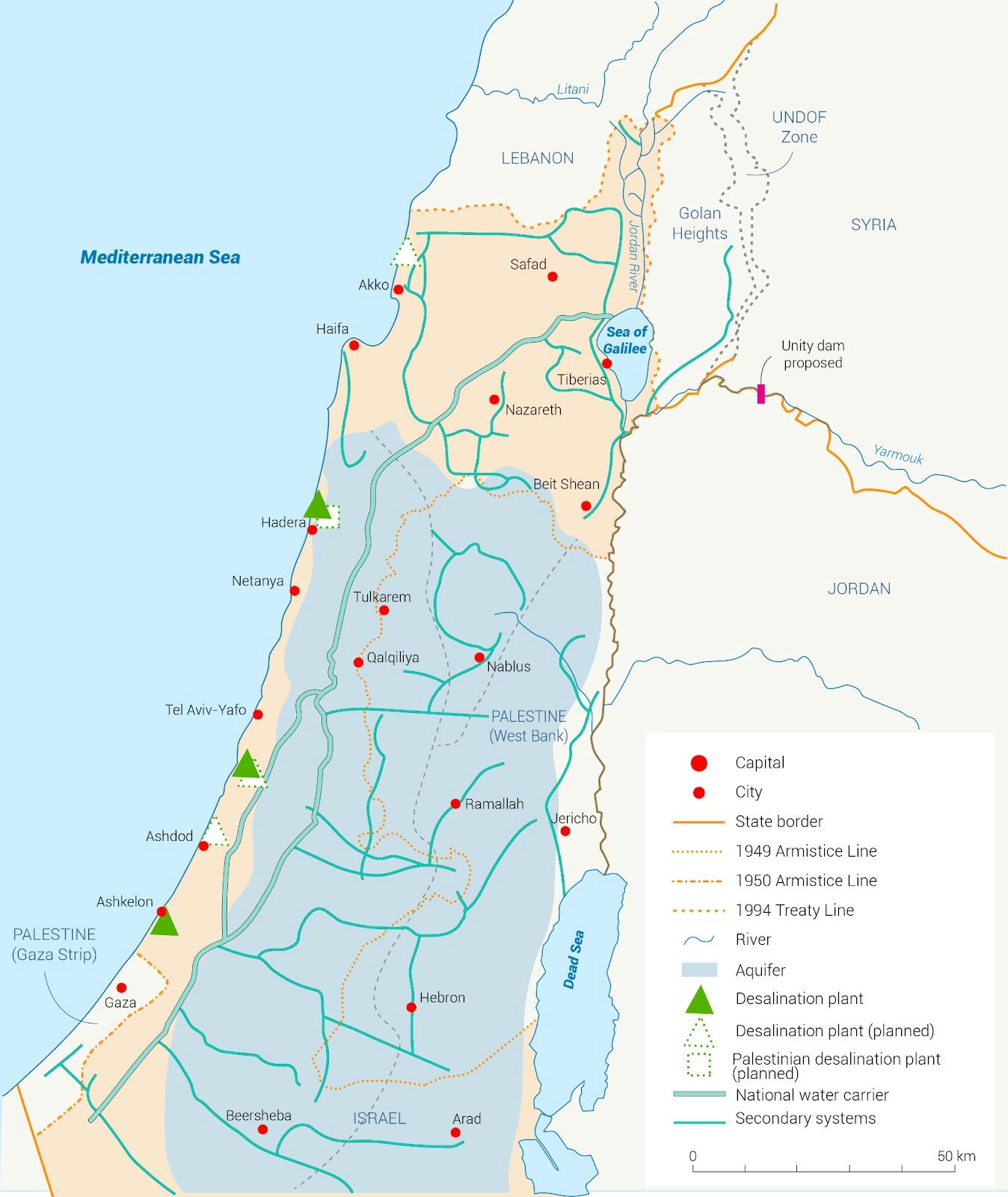
In the early 2000s, the Middle East was in the midst of a decade-long drought. Wells ran dry as millions of farmers across Syria, Lebanon, and Jordan lost their crops and their livelihoods. Israel, too, was nearing a point of no return. The country sourced most of its water from the Sea of Galilee. By 2008 it had dried up so much that it was rapidly approaching its “black line,” a threshold below which the river would be irreversibly damaged by salt infiltration.
Fortunately, Israel was ready. It had known for years that the Sea of Galilee alone would not be sufficient to support its growing population and industry. So in 1997, a year before one of the worst droughts in history hit the Middle East, the Israeli Water Authority conceived of a Desalination Master Plan that aimed to provide drinkable water on a large scale to the region.
Israel sits right beside the Mediterranean Sea. Thus, the plan envisioned the construction of a row of seawater desalination plants all along the Mediterranean coast which would convert enough seawater into fresh water to supply the entire country with abundant water forevermore. The plants would be connected to Israel’s national water carrier system, thereby bringing pure water directly to consumers. It would even be able to replenish depleting sources like the Sea of Galilee.
Source: Fanack Water
Over the following decade, Israel built five massive desalination plants in Ashkelon, Palmachim, Hadera, Sorek, and Ashdod. Today, these desalination systems supply nearly all of the country’s tap water. The national emphasis on desalination technologies incentivized research and company formation, leading to increasingly efficient desalination processes. In time, Israel even became a net exporter of water and began selling its surplus to neighboring countries. By 2022, the Sea of Galilee was at its highest level in thirty years and Israel became a producer of the cheapest desalinated seawater on Earth.
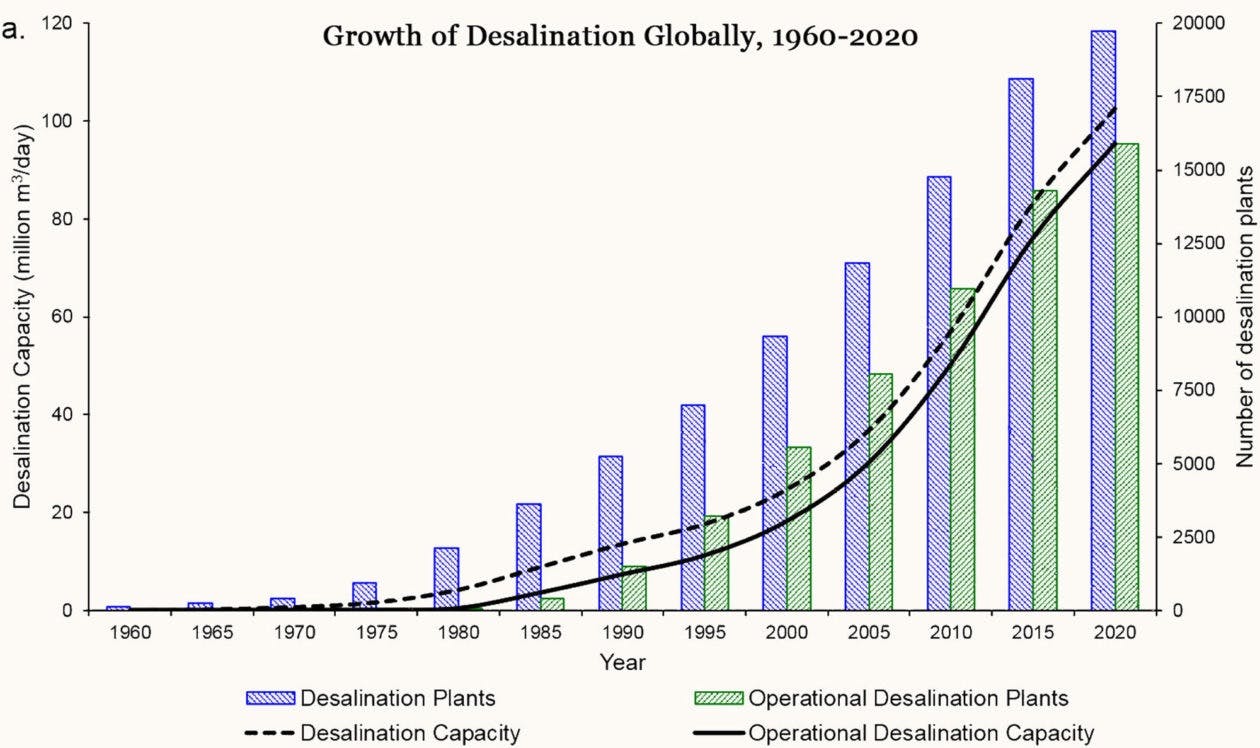
In time, desalination became an increasingly prevalent source of water in the Middle East, where the technology has come into its own. There are now nearly 20,000 desalination plants in operation around the world. However, more than half of the world’s desalination capacity is in the Middle East, where the largest plants are located in the United Arab Emirates, Saudi Arabia, and Israel, which supply nearly all of the region’s drinking water.
As water scarcity now rears its head in the United States, and organizations like NASA claim that 11 trillion gallons of water will be needed to replenish the water lost in California’s historic drought, while another 10 trillion will be needed to replace the water lost from the drying Colorado River, many are pointing to desalination as an obvious solution to this problem on a large scale.
The Origins of Desalination
The basic principles of desalination have been with us for millennia. Aristotle may have been describing the practice of distillation in writing that “salt water, when it turns into vapor, becomes sweet, and the vapor does not form salt water again when it condenses. This I know by experiment.” Distillation is the process of boiling sea water to separate water from salt and collecting the cooled, desalinated water.
Distillation was later used on ships in the 17th century to provide potable water fit for human consumption to voyagers embarking on month-long journeys across the Atlantic. Sailors would equip their vessels with large evaporators that boiled ocean water and distilled it down to water they could drink. Though thermal desalination like this was a good solution in extreme circumstances, the evaporators worked slowly and required a large amount of thermal energy relative to the potable water they ultimately produced.
However, for centuries, boiling water remained the only effective way to desalinate water. In 1930, one of the first desalination plants was constructed on the island of Aruba, then still a colony of the Dutch. Caribbean islands traditionally used wells or collected rainwater for drinking water, but given Aruba’s quickly growing population, another source of water was essential. In the 1950s, as the price of oil reached a historic low, thermal desalination seemed viable for the first time. In 1951, Kuwait built the first seawater desalination plant in the Middle East.
The United States, facing an extended drought in the Southwest during the 1950s, was similarly enamored by the technology. By 1955, the US established the Office of Saline Water, which within a few years helped usher in the construction of a multi-stage flash distillation plant in Freeport, Texas in 1961. Multi-stage flash distillation improved the efficiency of traditional thermal distillation plants by using a series of successively lower pressure stages, allowing seawater to boil at lower temperatures and requiring less energy for vaporization.
At the opening of the desalination plant, President John F. Kennedy said that desalination was “a work that in many ways is more important than any other scientific enterprise in which this country is now engaged.” A remarkable statement to make during the space race!
The nationwide emphasis on desalination in the US led to a very fruitful era for desalination research. Attempts were made to find more energy-efficient methods of removing salt content from water. Rather than boiling the freshwater– as with both multi-stage flash distillation and multiple-effect distillation – researchers were curious if there was a viable way to filter salt directly out of the water in a way that required less energy consumption. In 1965, researchers at the University of California succeeded in developing one of the first synthetic membranes capable of doing just this. That same year, a desalination plant in Coalinga, California was opened to desalinate brackish groundwater using the filtering membranes.
Eventually, as the Southwestern drought ended, interest in desalination fell away. By 1974, the Office of Saline Water was subsumed under the larger Office of Water Research and Technology, which itself was ultimately shuttered in 1982. However, even as America bowed out, the filtration and membrane distillation technology pioneered by the United States was picked up elsewhere. The broader Middle East and Israel in particular improved upon American designs and found clever ways to improve on energy efficiency and cost around the margins.
The ensuing period led to a desalination boom. If only 8,000 cubic meters were being produced by seawater desalination plants per day in 1970, today over 97 million cubic meters of freshwater are output by desalination plants each day.
Source: Environment 360, Yale
How Desalination Works
Though distillation technologies are still used at some desalination plants across the world, membrane filtration-based desalination is state-of-the-art in the present day. This process is employed at what is referred to as a reverse osmosis desalination plant.
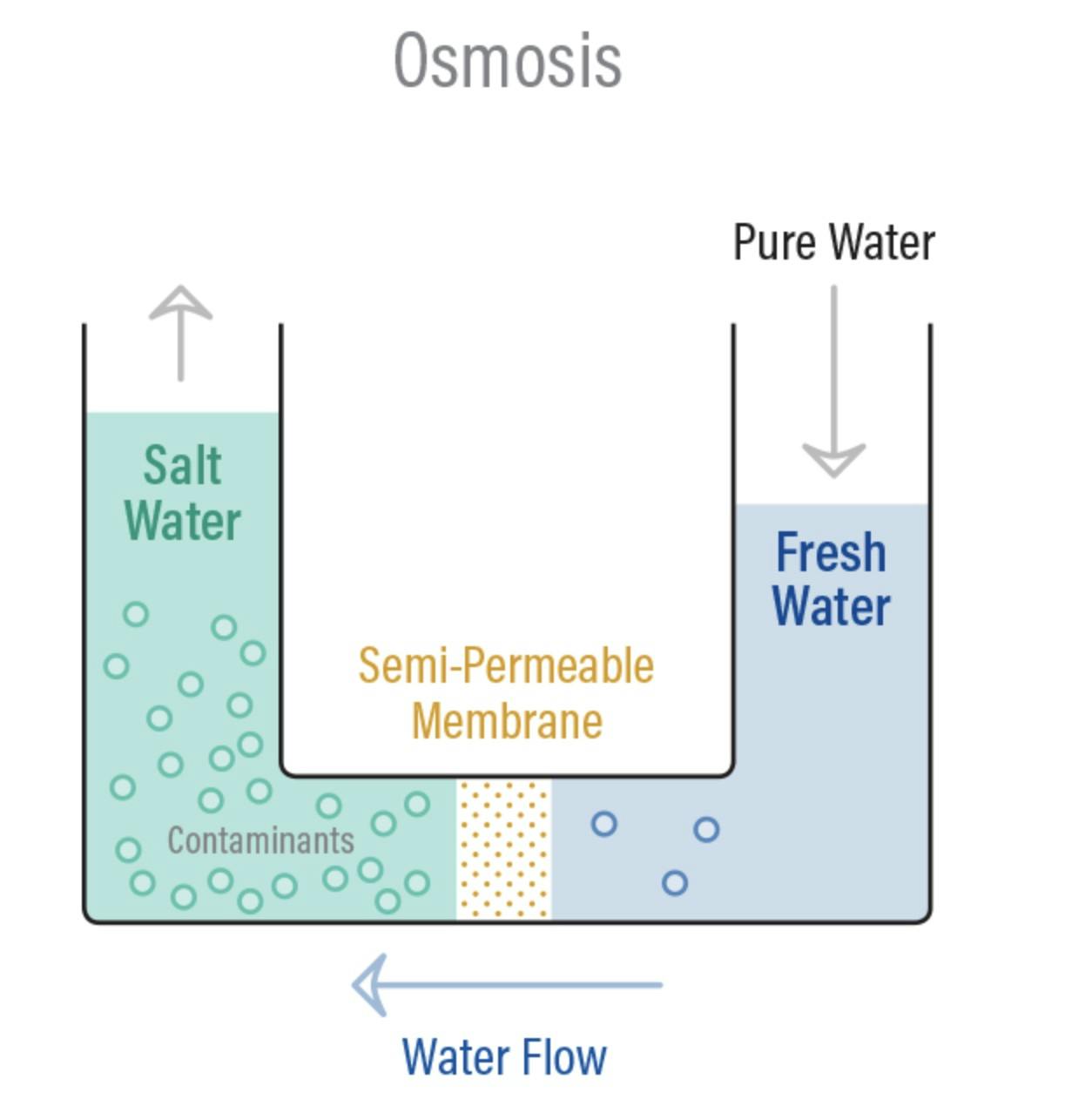
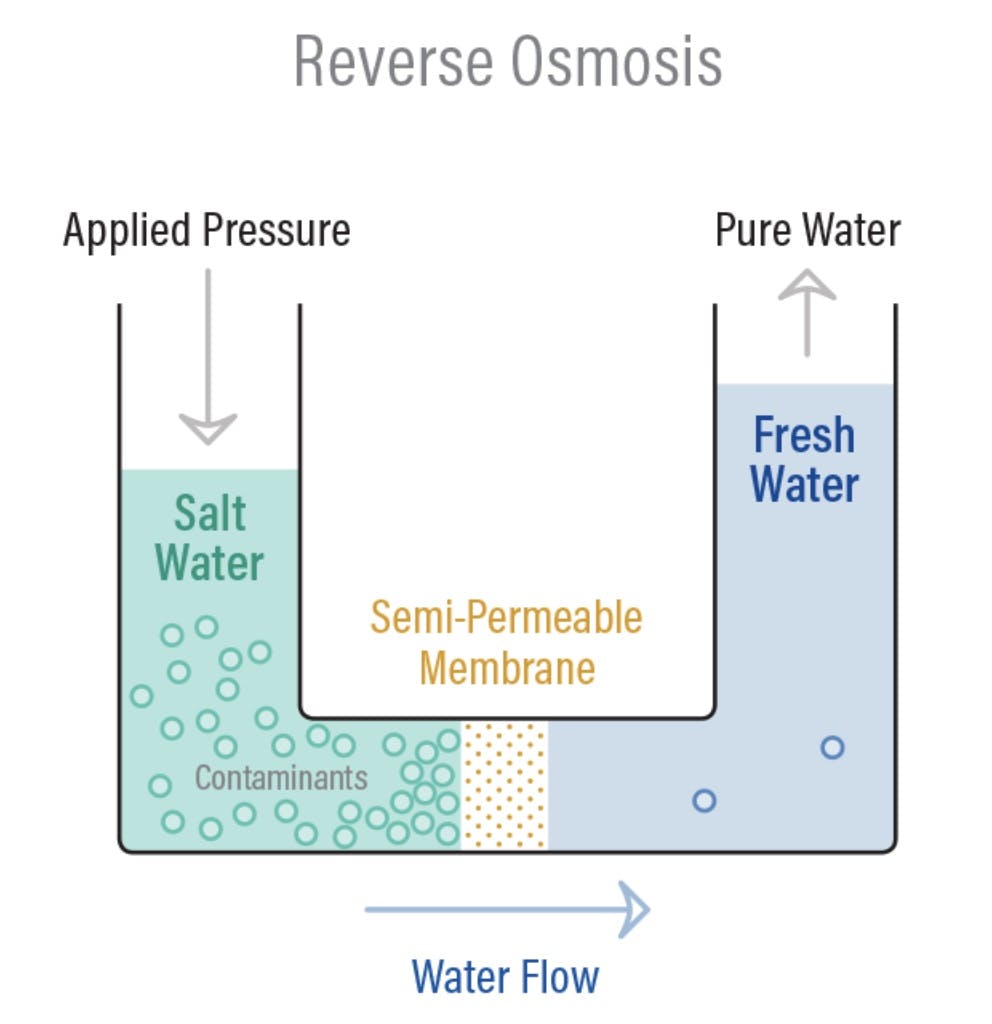
Osmosis is a naturally occurring phenomenon that happens all the time, all around us. Essentially, it’s a process where water moves from less concentrated solutions to more concentrated ones when separated by semipermeable membranes. This movement from an area of low solute concentration to an area of high solute concentration is sometimes referred to as forward osmosis. Osmosis is how plant roots absorb water from soil, and how our kidneys absorb water from our blood to filter it!
Source: PureTec
Reverse osmosis is simply this operation in reverse. Instead of producing two equally concentrated solutions separated by a membrane, the goal of reverse osmosis is to use a membrane that separates a solution that gets more concentrated over time from a solution that gets less concentrated over time. Under normal circumstances, the more diluted solution would flow across the membrane to the more concentrated side. To induce reverse osmosis, pressure needs to be applied to get the concentrated solution to flow in the opposite direction.
Source: PureTec
Applying this pressure uses energy, which is why the reverse osmosis desalination process is energy-intensive. In general, the more concentrated the solution is, the more pressure will need to be applied to push it across.
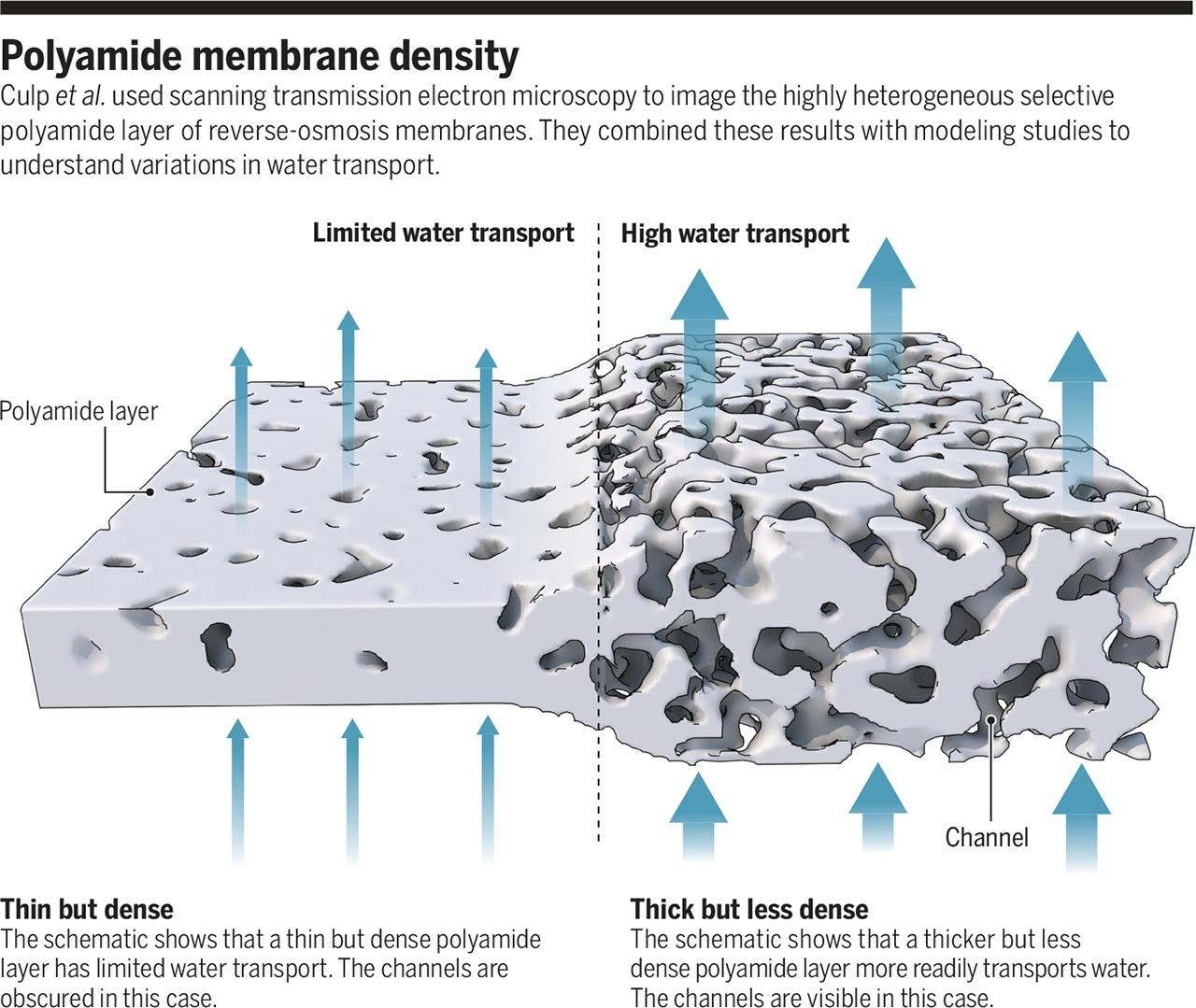
The membranes used in reverse osmosis today are called polyamide membranes. They are synthesized from chemical compounds containing nitrogen and a compound called trimesoyl chloride which contains carbon, oxygen, and chlorine atoms. When these compounds react, they link together in a tight structure with pore-like openings that allow tiny molecules like water to pass through, but little else. Salt ions, like sodium (Na+) and chloride (Cl-) are left out.
Source: Science
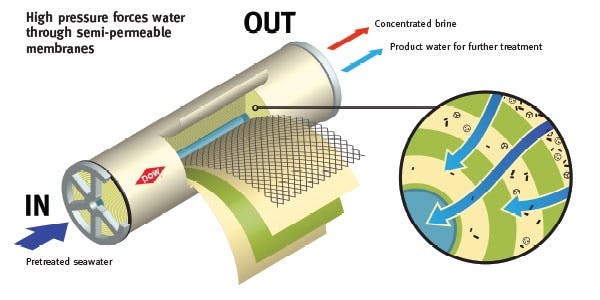
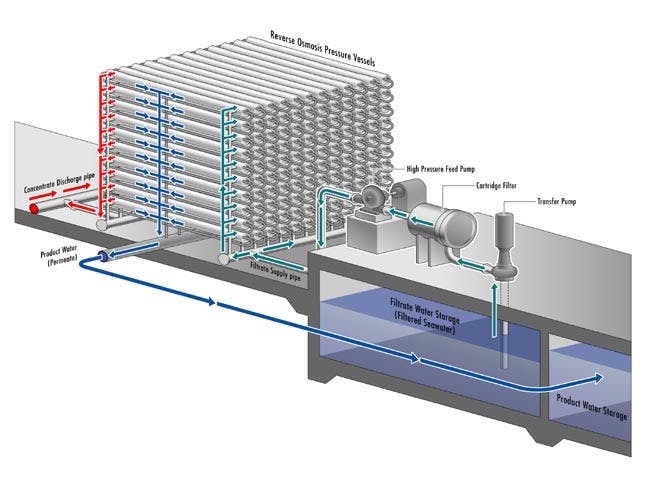
The membrane material is tightly wound within elongated tubes so that each unit of water interacts with the largest surface area of membrane possible. In a typical seawater reverse osmosis plant, there are multiple stages of pressurized filtering tubes through which water is piped. No stage is perfectly efficient, meaning that not all of the input water fed through the tubes comes out perfectly desalinated. This is why stages are necessary. Whereas the first stage can extract around 65% of potable water from the total amount fed in, the remainder will move on to further stages which can run it through the reverse osmosis process again to filter it down even more.
Source: Carlsbad Desal
In total, modern desalination plants are capable of converting roughly 80% of the saline water piped into potable water. The remainder is so heavily concentrated with salt that filtering it even further would be economically inefficient. This remainder is called brine and needs to be disposed of appropriately. An additional consideration for the modern reverse osmosis desalination plant is that the reverse osmosis membranes (RO membranes) need to be maintained and cleaned periodically as molecular compounds and minerals can get stuck in the membrane, decreasing its efficiency.
Source: Carlsbad Desal
The largest desalination plant in the world, the Ras Al-Khair complex in Saudi Arabia, is capable of producing up to a million cubic meters of water per day. Today, the most efficient plants can do so with an energy efficiency of 2.5 to 3.5 kWh per cubic meter. In itself, that’s a significant improvement over plants from the 1970s, which could only attain a threshold of 7 to 9 kWh per cubic meter of water output — and those were the reverse osmosis plants. Traditional thermal distillation plants still require anywhere between 7 to 12 kWh of energy per cubic meter of water produced.
There is a theoretical limit to how efficient reverse osmosis can be. Essentially, the thermodynamic limit states that the minimum amount of energy required to desalinate a cubic meter of seawater is roughly 1 kWh per cubic meter — so there’s still room for improvement given current designs. However, even at current electrical energy requirements, the amount of energy needed to produce a cubic meter of water via reverse osmosis is not all that unreasonable.
Given that the average American household consumes somewhere around 100,000 gallons of water per year, the total electrical energy required to desalinate that much water through reverse osmosis would be something like 1,135 kWh per year. That’s less than the average annual energy consumption of the typical refrigerator — which sits somewhere around 1,400 kWh.
Desalination Going Forward
Desalination is an important consideration for the future of water security. Initially, in the 1950s, the technology only gained attention after energy prices plummeted sufficiently to make it feasible. In the current era, as renewable energy capacity expands and energy demands from electric vehicles and increased domestic manufacturing capacity ask more of the grid, desalination is increasingly viable from an economic perspective.
Desalination, however, is really only feasible in areas with easy access to abundant sources of saline water because the cost of transporting water is another limiting factor. States like California, whose population hubs sit on the Pacific Ocean coast, could greatly benefit from expanded desalination infrastructure à la Israel. Coastal cities like Los Angeles already import nearly 85% of their water supply anyway, and could clearly benefit from expanded production of desalinated water.
California is well aware of these issues. In 2022 it published a Water Supply Strategy which aims to increase the supply of desalinated water, though not at the large scale seen in the Middle East. The state claims that doing so would be difficult anyway, since coastal real estate is protected, and must abide by the terms of California’s Coastal Act. California already has five desalination plants on its coast, including one in San Diego, Santa Barbara, San Luis Obispo, and two around Monterey. Many of these show promise — Santa Barbara’s Charles E. Meyer desalination plant already supplies a third of the city’s water needs.
The outstanding issues with desalination concern the disposal of brine. In general, most brine is simply disposed of into the source where it came from, usually the nearest ocean or sea. In the Gulf region, where most of the domestic drinking water is supplied by desalination plants, there are growing concerns that saline coasts surrounding the Persian Gulf are becoming more concentrated due to increasing brine discharges from desalination plants.
However, there are still valuable minerals and elements to be extracted from the brine itself. Undoubtedly, the extraction of these minerals would also require energy consumption and money, though a paper from the International Journal of Water Resources Development made the fascinating observation that “…if salt is extracted from the brine from all desalination plants that are currently in operation, and this is practiced universally, it would mean that the current global production of salt will rise by over 10 times.”
The thermal processes of distillation and reverse osmosis are not the sole means of conducting desalination, and other techniques are under development. As with many things, we can look to nature for examples of alternative designs. For instance, biologists are hard at work examining how anadromous species of fish — like salmon or eels, which are born in freshwater but mature in oceans — desalinate water in their bodies. Mangroves are an example of plants that have desalination systems embedded into their roots.
If biomimicry doesn’t pan out, others are investigating how advanced nanotechnology could help produce more selective membranes. In general, producing membranes with higher selectivity would improve the efficiency of reverse osmotic membrane processes by requiring fewer stages of filtration, decreasing the amount of energy required to produce a cubic meter of potable water.
In any event, desalination technology can only continue growing as a locus of attention as cheap, renewable energy expands and our dexterity in material science improves. The availability of our water supply depends on it.
BLOG
The Growing Importance of Desalination
August 26th, 2024
August 26th, 2024
Via Contrary, a deep dive into the booming desalination industry:
This entry was posted on
Monday, August 26th, 2024 at
6:53 am and is filed under
News. You can follow any responses to this entry through the
RSS 2.0 feed.
Both comments and pings are currently closed.
© 2025 Water Politics LLC . 'Water Politics', 'Water. Politics. Life', and 'Defining the Geopolitics of a Thirsty World' are service marks of Water Politics LLC.